As an organic chemistry tutor, there are a couple of questions that I get asked about a lot. “What is the difference between the nucleophile and the base? “How can I use this to determine substitution and elimination reactions?”
Definitions
The first thing that I want to let you know is that a nucleophile and a base are usually the same things.
Nucleophile, as the name implies, has the terms “nucleo,” which means nucleus, and “phile,” which means loving. It gives us an obvious idea that nucleophile is a nucleus-seeking chemical species. And since the core is positively charged, a nucleophile is an atom or molecule that is of negative or partially negative charge. This way, it can attract the positivity of the nucleus.
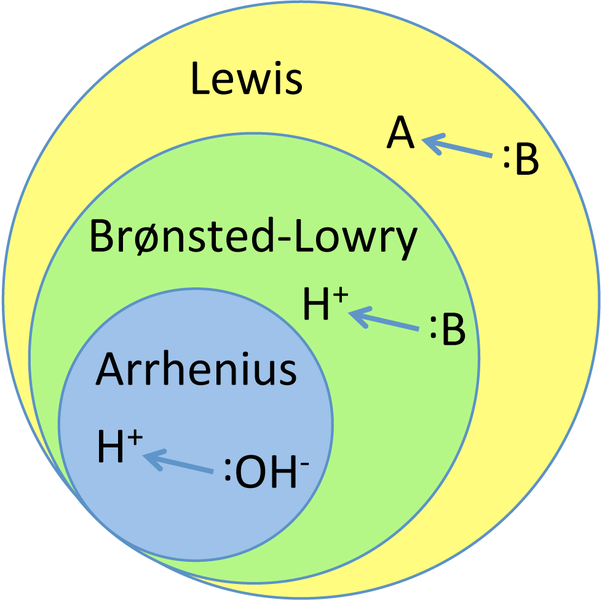
A base, on the other hand, is just like what you can see from the acids-and-bases theories of Arrhenius, Bronsted-Lowry, and Lewis. The Arrhenius theory defines base as a molecule that disassociates in water to give off an OH-. However, keep in mind that this will not always be the case in the substitution and elimination processes because your solvent will not always be water. Conversely, the Bronsted-Lowry theory asserts that a base is a proton acceptor that picks up an H+. The Lewis theory states that a base is an atom or molecule that is willing to donate an electron pair to form a bond.
Comparisons
Now that we know the three definitions for a base or a nucleophile, let’s compare them with each other. We can exclude the Arrhenius definition because we do not always use water as a solvent.
An OH-, a species that picks up an H+, and a species that donates a pair of electrons to form a bond; they all sound like the same thing, right? If a molecule wants to pick up a proton, it will “offer” a set of electrons to pick up that proton or that H+. If the proton is H+, we can assume that the base is going to be negatively charged.
Can you see the pattern here? I am not trying to burst your bubble or blow your head off; I want to show you that the nucleophile and the base are more or less the same things. So, the next question is, “How do we tell them apart?”
Contrasts
It’s all about the context. If you are still getting confused, try to understand this non-complex example: John has a sister, Mary, and a large family. To Mary, John is a brother; to his mother, John is a son. Let me ask you, is John a brother or a son? The answer is that he’s both. It just depends on the context that you’re using this.
If a molecule participates in nucleophilic substitution, we call this attacking molecule as the nucleophile. If a unit takes part in beta elimination, we call this as the base. But even if it has been established above that finding out whether your nucleophile or base is undergoing substitution or elimination reaction is essential, there are still two other reactions that you should look out for: the 1-type and 2-type reactions.
1-Type Reaction
The 1-type Reaction refers to SN1 and E1 reactions. They make use of carbocation intermediates and a fragile attacking molecule; that’s why we cannot expect to see a fast response with this. It just keeps on waiting for this carbocation to form before it can proceed with the Reaction.
2-Type Reaction
As for the 2-type Reaction, it refers to SN2 and E2 reactions and has a potent attacking molecule. The latter can be thought of as the bully molecule because it doesn’t want to wait for a carbocation to form; it just goes and attacks directly.
For you to be able to tell these reactions apart, you have to look at the chemical equation. Ask yourself, “Is there a negatively charged molecule here or not?”
A negative charge is what’s going to clue you in at this point. You are looking at an active molecule, so it must be a 2-type reaction. Examples include OH-, SH-, CH3O-, CH2CH3O-, and so on.
Alternatively, the 1-type Reaction is likely to have the neutral version of our strong nucleophiles and bases. H2O, H2S, CH3OH, and CH2CH3OH are just of the examples for this.
This is not so difficult to understand now, is it?